Gurdasani et al. 2019
Authors: Deepti Gurdasani, Tommy Carstensen, Segun Fatumo, Guanjie Chen, Chris S Franklin, Javier Prado-Martinez, Heleen Bouman, Federico Abascal, Marc Haber, Ioanna Tachmazidou, Iain Mathieson, Kenneth Ekoru, Marianne K DeGorter, Rebecca N Nsubuga, Chris Finan, Eleanor Wheeler, Li Chen, David N Cooper, Stephan Schiffels, Yuan Chen, Graham R S Ritchie, Martin O Pollard, Mary D Fortune, Alex J Mentzer, Erik Garrison, Anders Bergström, Konstantinos Hatzikotoulas, Adebowale Adeyemo, Ayo Doumatey, Heather Elding, Louise V Wain, George Ehret, Paul L Auer, Charles L Kooperberg, Alexander P Reiner, Nora Franceschini, Dermot P Maher, Stephen B Montgomery, Carl Kadie, Chris Widmer, Yali Xue, Janet Seeley, Gershim Asiki, Anatoli Kamali, Elizabeth H Young, Cristina Pomilla, Nicole Soranzo, Eleftheria Zeggini, Fraser Pirie, Andrew P Morris, David Heckerman, Chris Tyler-Smith, Ayesha Motala, Charles Rotimi, Pontiano Kaleebu, Ines Barroso and Manj S Sandhu
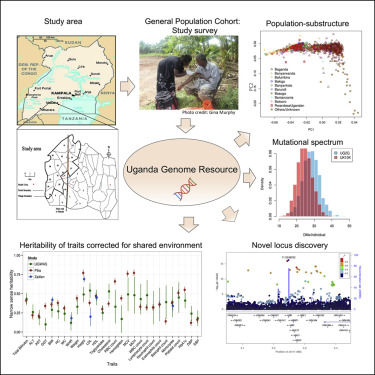
Abstract: Genomic studies in African populations provide unique opportunities to understand disease etiology, human diversity, and population history. In the largest study of its kind, comprising genome-wide data from 6,400 individuals and whole-genome sequences from 1,978 individuals from rural Uganda, we find evidence of geographically correlated fine-scale population substructure. Historically, the ancestry of modern Ugandans was best represented by a mixture of ancient East African pastoralists. We demonstrate the value of the largest sequence panel from Africa to date as an imputation resource. Examining 34 cardiometabolic traits, we show systematic differences in trait heritability between European and African populations, probably reflecting the differential impact of genes and environment. In a multi-trait pan-African GWAS of up to 14,126 individuals, we identify novel loci associated with anthropometric, hematological, lipid, and glycemic traits. We find that several functionally important signals are driven by Africa-specific variants, highlighting the value of studying diverse populations across the region.